An Atom Is Composed of a Very Dense Central
Not only is the nucleus very small but it also contains most of the mass of the atom. Opposite the gold foil is a screen that emits a flash of light when struck by a particle.

Atomic Theory Atoms Molecules Quiz Quizizz
In fact for all practical purposes the mass of the atom is the sum of the masses of the protons and neutrons.

. He also concluded that the electrons orbit the. No it is a molecules composed of a nitrogen atom bonded to three hydrogen atoms. It is composed from protons and neutrons.
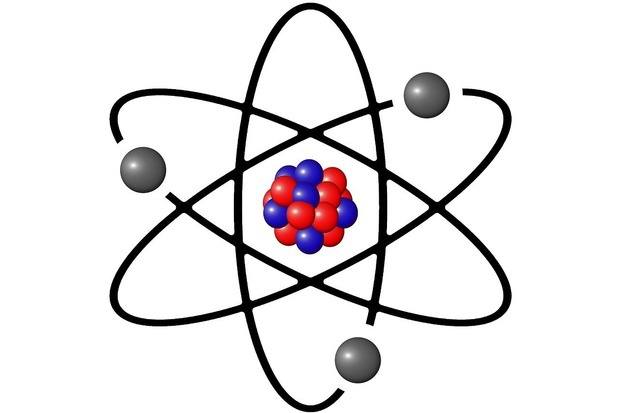
Nucleus protons no A particular isotope of an element is represented by the symbol 36sub17Cl. The extremely dense nature of the nuclei of atoms d Robert Millikan. The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons.
Most of the atom essentially empty A few particles deflectedsome straight back. A Nucleus B Protons C Zero. A The atoms of very few elements contain neutrons in their nuclei.
A very small part of the atom is very dense impenetrable. The nucleus that dense central core of the atom contains both protons and neutrons. The nucleus is very very small and very very dense when compared to the rest of the atom.
4 What is the center of the atom called quizlet. Crowded nucleus nuclear glue The protons of an atom are all crammed together inside the. What information is contained in a chemical formula.
The nucleus is very very small and very very dense when compared to the rest of the atom. Each element in a given compound comprises a fixed fraction or percent by __A__ of the compound. The mass must be concentrated.
6 What is the central core of the atom which makes up most of the atom mass. Protons have a positive charge neutrons have no charge and electrons have a negative charge. The nucleus of an atom contains neutrons and protons.
3 What is the core of an atom composed of. What is the dense central part of an atom. The nucleus of the atom is positively charged with the strength of this charge equal to the atomic number.
The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons except in the case of hydrogen-1 which is the only stable nuclide with no neutrons. 5 What did James Chadwick discover. Rutherford concluded from his metal foil experiments that most of an atom is empty space with a tiny dense positively charged nucleus at the center that contains most of the mass of the atom.
An atom is composed of a very dense central _____containing _____ which are positively charged and neutrons which have _____electrical charge. Ratio of elements in a compound 2. Electrons are outside the nucleus in energy levels.
The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons except in the case of Hydrogen-1 which is the only stable isotope with no neutron. The dense central portion of an atom is called the nucleus. An atom is composed of a very dense central __A__ containing __B__ which are positively charged and neutrons which have __C__ electrical charge.
9 What is the center of. Most of the mass of the atom is concentrated in a very small dense central area called the nucleus which is about 1100000 the diameter of the atom. Rutherfords atomic model became known as the nuclear model.
8 What is at the center of every atom Quizizz. The charge on an electron 6. The nucleus is the tiny dense central core of the atom and is composed of protons and neutrons.
The atomic nucleus is the small dense region consisting of protons and neutrons at the center of an atom discovered in 1911 by Ernest Rutherford based on the 1909 GeigerMarsden gold foil experiment. Nucleus protons neutral An element is the simplest form of matter that has unique _______ and chemical properties. After the discovery of the neutron in 1932 models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg.
An atom is composed of a very dense central nucleus containing _____ which are positively charged and neutrons which have _____ electrical charge. In the nuclear atom the protons and neutrons which comprise nearly all of the mass of the atom are located in the nucleus at the center of the atom. This observation is summarized by the law of definite __B__.
Which is a possible explanation for why the neutron was the last of the three fundamental subatomic particles to be discovered. An atom consists of a very dense central nucleus containing protons and neutrons with electrons moving about the nucleus at a relatively large distance from it. The size of nucleus will be proportional to the of highly scattered particles.
7 What is the particle that moves around the central part of the atom. Is ammonia an atom. An atom is composed of a very dense central _____ containing _____ which are positively charged and neutrons which have _____ electrical charge.
The center of an atom is called nucleus. Types of elements present in a compound. The rest of the atom is apparently empty space.
The atom is a basic unit of matter consisting of a dense central nucleus surrounded by a cloud of negatively charged electrons. Rutherfords diffraction experiment tests diffraction via a thin foil made of gold metal. The passing of many of the particles through suggested the.
Protons are positively charged neutrons have no charge electrons are negatively charged Protons and neutrons have roughly the same mass which is about 1840 times greater than the mass of an electron.
Topic 7 Lesson 1 Atomic Theory Other Quiz Quizizz

Reference Introduction To Atomic Structure Atomic

A Flow Chart Shows The Hierarchy Of Living Organisms From Smallest To Largest This Hierarchy Includes 1 An At Science Cells Science Biology Teaching Science
No comments for "An Atom Is Composed of a Very Dense Central"
Post a Comment